
Francis S. Collins, MD, PhD, Director of the National Institutes of Health
NIH Director Francis S. Collins, MD, PhD, said in yesterday’s opening session that he’s got a “smorgasbord of issues” he’s been wanting to discuss with anesthesiologists.
A smorgasbord indeed. In what he dubbed a “romp through the NIH,” Dr. Collins walked the audience through NIH biomedical research updates in categories that included advancing neurotechnology, the opioid crisis, the need for a more diverse and innovative research workforce, and COVID-19.
Naturally, the immediacy of the COVID-19 pandemic affects all of the above, and the NIH is throwing its full weight into getting the United States – and the globe as a whole – the help that it desperately needs.
“Unless something is done,” said Dr. Collins, “this has the potential to go on and on for a very long time, taking too many lives. In diagnostics, therapeutics, and vaccines, we are all in.”
COVID-19 research updates, which are the combined work of both public and private entities, are threefold: therapeutics for the treatment of the disease, vaccines for the prevention of the disease, and rapid testing so that we have the capability to identify the magnitude of the disease.
Three kinds of therapeutics – immune modulators, monoclonal antibodies, and antithrombotics – are currently in Phase 3 of patient trials, while two other promising treatments are in Phase 2. Dr. Collins said he has high hopes where all are concerned.
“All are either under way or about to get under way at a remarkable pace,” he said. “There has never been one at this speed before.”
As far as vaccines go, there are currently six candidates already in Phase 3 trials or will be soon.
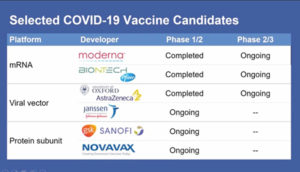
“People want to know: When will we have a vaccine that’s been proven safe and effective? That takes time,” he said, noting that the AstraZeneca trial is currently on hold while researchers attempt to determine whether an adverse event on a patient in Britain is related to the vaccine or an isolated event. Each vaccine except the one being developed by Janssen will require two doses, said Dr. Collins, and then there will be a period of watching trial participants to see if there are adverse events reported.
“Even for the most optimistic person looking at timetables, it seems pretty unlikely that we would have a readout of these trials before November or December,” he said, “and I would say December is more likely.”
He also emphasized the importance of listening only to the parties responsible for monitoring the safety and efficacy of the vaccines. “They’re the only ones who can see the unblinded data while the trial is moving forward. Until then, we watch and wait – and that’s the way it should be.” With admittedly cautious optimism, he said there’s a fair chance that more than one of the vaccines will work, and that preparations are already being made to be able to produce a successful vaccine as quickly as possible – but it’s not the kind of process where you can cut corners.
“We want the public to be totally confident that if something is said to be safe and effective, it really is,” said Dr. Collins.
The final piece of the COVID-19 response puzzle is testing. The NIH is working to accelerate the innovation, development, commercialization, and implementation of COVID-19 tests to expand the capability to identify the disease.
“If we really want to see control of COVID-19,” said Dr. Collins, “it is critical to find out who is infected quickly. You can’t count on people turning sick to be able to assess who is spreading the virus. Those asymptomatic people can be spreading the virus quite quickly.”
Dr. Collins ended his presentation with words of thanks and encouragement to anesthesiologists.
“We think of you as our partners,” he said. “We count on you to be the place that most of the really exciting ideas or coming from.” As for COVID-19, “We’re going to get through this,” he said. “Not right away, but we will.”

Ambassador Deborah L. Birx, MD, the White House Coronavirus Response Coordinator
Following Dr. Collins’ talk, Ambassador Deborah L. Birx, MD, the White House Coronavirus Response Coordinator, joined the presentation from her home to thank anesthesiologists for their role in combatting the pandemic.
“At a time when we had less than 16,000 ventilators and we had a large need,” she said, “anesthesiologists helped and made innovation possible.” Dr. Birx worked closely with ASA throughout the onset of the pandemic to learn how to transform ventilators for COVID-19 patients, and despite the increased number of machines, anesthesiologists still have a critical role to play.
“That doesn’t change the fact that we need people who know how to utilize them and how to support others utilizing them,” she said. “I want to thank the anesthesiologists who came out of their ORs and supported us. We will probably need that again – and I hope we don’t – but we will probably need that kind of direct patient care support again. We do have enough ICU beds and we do have enough ventilators; what we don’t have is enough human capacity to ensure that every client is served.”
Return to Index